Multi-Pathogen Vaccine Kit
AIVITA’s patient-specific vaccine platform has also been used to create treatments for multiple areas of cancer, with promising safety and efficacy reported to date.
Clinical Studies
AIVITA investigated this vaccine approach in COVID-19 in a 180-participant Phase 2 clinical study in Indonesia, which followed a Phase 1 clinical study showing highly favorable safety and preliminary efficacy. Published results from those studies demonstrated a 96% T-cell response and far superior and longer-lasting protection compared to mRNA vaccines.[1] Notably, our vaccine provides superior protection well beyond 12 months, with the fewest and lowest adverse events of any available vaccine.[2] Importantly, these antigen-loaded dendritic cells mounted an immune response in lymphocytes from the same patient, showing immune activation from the vaccine.
A key benefit of AIVITA’s approach is its potential for rapid scalability and mass distribution. Given the right tools, any one of thousands of minimally-equipped laboratory settings nationwide, or worldwide, could manufacture the patient-specific vaccine using AIVITA’s proprietary reagents.
Dendritic cell-based treatments have been widely studied in the clinical setting, showing strong safety and antigen-specific efficacy for creating an adaptive immune response.
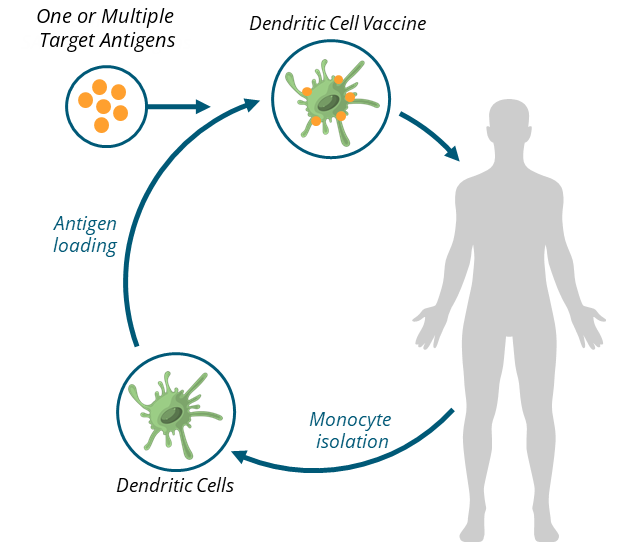
Each vaccine is personalized to the individual receiving it, using equipment and methods widely available in clinics worldwide.
- The process begins with a blood draw
- The blood is enriched for monocytes, a type of white blood cell that plays a role in innate immune response
- The monocytes are differentiated into dendritic cells, an immune messenger cell
- One or multiple target antigens are loaded onto the dendritic cells
- Following incubation, the vaccine is ready for administration
Phase 1 (Indonesia): NCT04690387
Phase 2 (Indonesia): NCT05007496
Publications:
- Dillman RO, Nistor GI, Jonny J, Yana ML, Langford JL, et al. (2023) Prevention of Symptomatic Covid-19 Infection by Personal Dendritic Cell Vaccine. J Vaccines Immunol 8: 189. DOI:https://doi.org/10.29011/2575-789X.000189
- (2022) A personal COVID-19 dendritic cell vaccine made at point-of-care: Feasibility, safety, and antigen-specific cellular immune responses, Human Vaccines & Immunotherapeutics, DOI: 10.1080/21645515.2022.2100189
- Robert O Dillman, Gabriel I Nistor, Aleksandra J Poole, A Novel Vaccine for The Novel Corona Virus. Am J Biomed Sci & Res. 2020 – 11(3). AJBSR.MS.ID.001631. DOI: 10.34297/AJBSR.2020.11.001631
