Cancer Immunotherapy
“AIVITA’s approach aims to use the patient’s own immune system to seek out and destroy cancer cells, including the tumor-initiating cells that are responsible for the growth and spread of the disease.”
Overview
AIVITA’s immunotherapy technology creates personalized vaccines for cancer patients that target and eliminate tumor-initiating cells which are the seed of the disease. Used alone or in parallel with other treatments, AIVITA’s cancer immunotherapy represents a powerful new approach in the search for curative treatments for cancer.
The Advantage of AIVITA's Approach
The advantage of AIVITA’s approach lies in our pan-antigenic approach – targeting all neoantigens, rather than a select few – to train the patient’s immune system to target tumor-initiating cells. By educating immune cells isolated from the patient’s own blood to recognize the complete and unique antigenic signature of the patient’s own cancer-initiating cells, the treatment is capable of seeking out and destroying cancer and overcoming mutations over time.
In addition, by exposing the patient’s immune system to a broader spectrum of antigens represented in the patient’s own cancer, it is believed that the patient’s immune system will now be able to identify mutated or dormant tumor-initiating cells.
AIVITA Biomedical is working to identify a surrogate, predictive marker of efficacy and is developing a potency assay for predictive analysis.
Cancer immunotherapies aim to teach the host’s own immune system how to seek out specific antigens associated with cancer to destroy it wherever it has spread. AIVITA’s patient-specific approach utilizes the complete and unique antigenic signature of the patient’s own tumor. AIVITA believes this proprietary process provides the immune system with a fuller range of antigens specific to each patient’s own cancer.
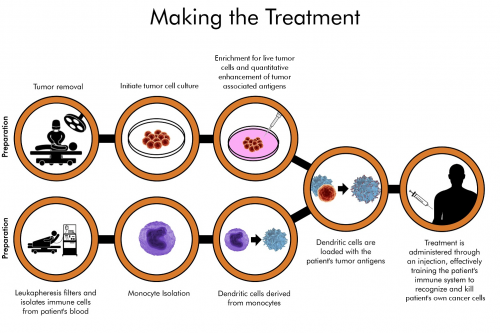
Development of the therapy begins when a sample of the patient’s surgically resected tumor is shipped to AIVITA Biomedical’s facilities. Cell culture allows for the enrichment and amplification of live tumor-initiating cells, both for the quantitative enhancement of the tumor-associated antigens and for the depletion of misleading signals that could interfere with the immunogenic process, such as dead cells, normal cells, and extracellular matrix. Using this approach, the patient’s tumor-initiating cells are enriched and become the target of AIVITA’s immunotherapeutic approach.
Through a process of blood filtration known as leukapheresis, antigen-presenting dendritic cells are derived from the patient’s blood. Dendritic cells, often referred to as the messenger cells of the immune system, are responsible for identifying foreign (non-self) antigens and presenting them as targets to the immune system. Using this to our advantage, we load the patient’s dendritic cells with the patient’s own tumor-associated antigens, priming them with the full antigenic signature.
Other approaches using a limited number of antigens, or those that attempt to prime dendritic cells in the presence of all tumor cells, have not shown sufficient efficacy to be meaningful cancer treatments. AIVITA’s approach primes the immune system more effectively to recognize the unique antigenic signature of the patient’s own seed of cancer, the tumor-initiating cells. Additionally, having educated the immune system to recognize tumor-initiating cells, AIVITA believes that any dormant cells which become active at a later time point will be similarly destroyed by the patient’s immune system.
Once prepared, the treatment is administered in a series of subcutaneous injections.