Multi-Pathogen Vaccine Kit
The arrival of personalized multi-pathogen vaccines
AIVITA Biomedical has harnessed its patient-specific vaccine platform to develop a rapidly scalable multi-pathogen kit for the prevention of infectious diseases. The resulting vaccine, comprised of the patient’s own antigen-presenting cells and antigens from multiple pathogens, minimizes adverse events and targets a range of infectious diseases to create adaptive immunity with a single low-cost shot.
*The personalized Multi-Pathogen Vaccine kit described here is not yet approved by the U.S. Food and Drug Administration and is not available for public sale. Personalized Multi-Pathogen Vaccines are experimental. Research is being conducted under IND regulations in the United States.
Configurable, multi-pathogen vaccines on demand
-
- The right vaccine modality for any occasion — Protect against endemic diseases of a region or population in a single shot. Keep pace with novel pathogens or mutating strains with a rapidly adaptable platform.
- Faster response times — Distributed scaled manufacturing at points-of-care enables new pathogen vaccines within days of sequencing the new pathogen antigen.
- Target: Adaptive immunity — Provides B cell and T cell antigen-specific immune responses shown to provide lasting adaptive immunity and protection.1
- Manufacturing — In-country vaccine manufacturing requires minimal technical skills and minimal facilities, while empowering vaccine independence of every country.
Broad pathogen coverage
AIVITA identifies and validates the optimal immunogenic antigens for each pathogen, for inclusion in or speedy updates to the vaccine kits, which enjoy a 3-year shelf life. The following pathogens have been shown to be effectively targeted:
In vitro validated T cell immune responses to the following pathogens:
Ebola, Mpox, Dengue, COVID-19, Influenza, Respiratory Syncytial Virus (RSV), Bacterial meningitis, Human Papillomavirus (HPV), Rabies, Herpes simplex
*All pathogens have been shown to elicit an appropriate immunological response in vitro, while safety and protective efficacy of this vaccine platform has been shown in clinical trials for some of the listed pathogens. Protective efficacy in human subjects must be explored in jurisdictions where the particular pathogen is prevalent.
Redefining pandemic preparedness
Shorter Development Time
Shorter development path to a viable vaccine. Begin vaccinating in days, not months or years.
Fewer Bottlenecks
Decentralized manufacturing makes for fewer bottlenecks in times of crisis.
Simplified Supply Chain
Ships at +4° C, exceeding the reach of ultra-cold shipped vaccines, especially in low-resource environments.
Parallel Deployment
The kit enables simultaneous decentralized roll-out of vaccine production, allowing for a vaccine that targets multiple pathogens in a single shot.
Unrestricted Scaling Potential
Decentralized manufacturing and distribution lends to unrestricted scale-up potential.
Keeping Pace with Mutations
Less downtime, no wasted vaccine lots when the virus mutates. Simply swap the antigen in the kit.
How it works
AIVITA’s multi-pathogen vaccine kit allows for the creation of a personal vaccine made from the subject’s own immune cells and target antigen.
- Vaccine creation starts with a small blood draw.
- Antigen-presenting cells are isolated from the subject’s blood sample.
- Target antigen(s) are introduced to the antigen-presenting cells.
- Following incubation and with minimal technical work, the personal vaccine is now ready for administration.
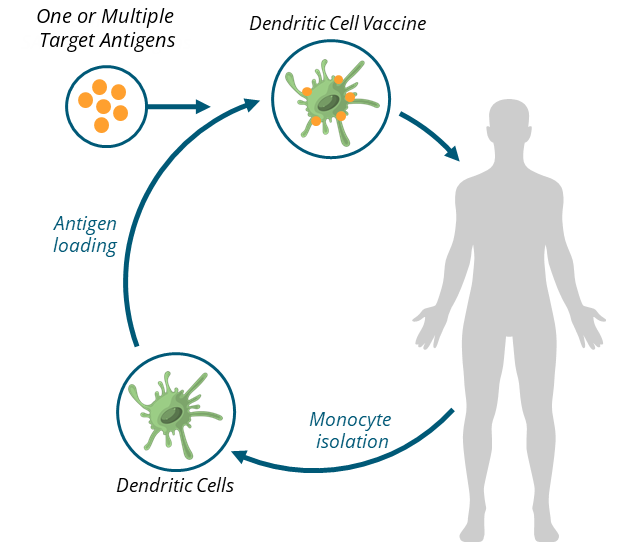
About dendritic cell personal vaccines
Dendritic cells direct the immune system to target the antigens they carry; as they can carry multiple antigens, they can instruct the immune system to attack multiple antigens. They capture antigens from pathogens and present them to T-cells, which are crucial for initiating a targeted immune response. In the context of vaccines, dendritic cells process the vaccine’s antigens and activate T-cells to create memory cells. These memory cells allow the body to recognize and quickly respond to the actual pathogen if encountered in the future. Dendritic cells play a key role in ensuring that vaccines effectively prepare the immune system for long-term protection.
Personal vaccines combine the antigens from one or multiple pathogens with dendritic cells derived from the patient’s drawn blood. The result is antigen-presenting dendritic cells that can be delivered as a vaccine, providing a more direct path to long-lasting adaptive immunity.
Clinical studies
AIVITA investigated this personal vaccine approach in COVID-19 in a 145-participant Phase 2 clinical study in Indonesia, which followed a 31-participant Phase 1 clinical study showing highly favorable safety and preliminary efficacy.
Published results demonstrated a 96% T-cell response and longer-lasting protection from symptomatic COVID-19 infection compared to mRNA vaccines.1 Notably, our vaccine provides superior protection for 12 months, with the fewest and least severe adverse events of any available vaccine.2 Importantly, these antigen-loaded dendritic cells mounted an immune response in lymphocytes from the same patient, showing immune activation from the vaccine.
Phase 1: NCT04690387
Phase 2: NCT05007496
Publications:
- Dillman RO, Nistor GI, Jonny J, Yana ML, Langford JL, et al. (2023) Prevention of Symptomatic Covid-19 Infection by Personal Dendritic Cell Vaccine. J Vaccines Immunol 8: 189. DOI: 10.29011/2575-789X.000189
- Gabriel I. Nistor, Robert O. Dillman, Rockelle M. Robles, James L. Langford, Aleksandra J. Poole, Muchlis A. U. Sofro, Yetty M. Nency, Jonny Jonny, Martina L. Yana, Mahammad Karyana, Endang S. Lestari, Ria Triwardhani, Mujahidah Mujahidah, Retty K. Sari, Nur A. Soetojo, Djoko Wibisono, Daniel Tjen, Taruna Ikrar, Gregory Sarkissian, Haryono Winarta, Terawan A. Putranto & Hans S. Keirstead (2022) A personal COVID-19 dendritic cell vaccine made at point-of-care: Feasibility, safety, and antigen-specific cellular immune responses, Human Vaccines & Immunotherapeutics, DOI: 10.1080/21645515.2022.2100189
- Robert O Dillman, Gabriel I Nistor, Aleksandra J Poole, A Novel Vaccine for The Novel Corona Virus. Am J Biomed Sci & Res. 2020 – 11(3). AJBSR.MS.ID.001631. DOI: 10.34297/AJBSR.2020.11.001631
